Meet The Health IT Vendors Working To End The Opioid Crisis
The U.S. is in the midst of a national crisis when it comes to opioid misuse and overdose. Last year, 52,000 people died from a drug overdose — 33,091 of which were caused by a prescription or illicit opioid, according to the Centers for Disease Control and Prevention.
And 12 million people misused opioids in 2016, SAMHSA found.
While the President proclaimed in August that he thought the opioid crisis was a national emergency, he’s not yet taken the necessary legal steps to actually make a difference in the uphill fight against the epidemic.
For example, a federal declaration would ease restrictions on who can prescribe medications used to treat opioid addiction, while providing extra funds from disaster relief. But while the White House is still processing an “expedited legal review,” there are health IT vendors pursuing a private sector effort to thwart the overprescribing and overdosing of opioids.
There are a variety of vendors in this space attempting to make an impact in the crisis, and we’ve highlighted those with the most unique or largest presence in the sector.
SPR Therapeutics: Tech in lieu of meds
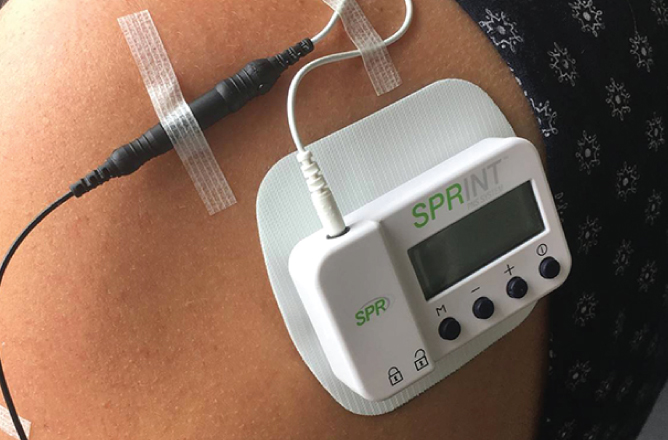
SPR Therapeutics’ SPRINT device is a drug-free wearable that uses a thread-like wire and wearable stimulator to alleviate pain. In doing so, the device could replace the need for opioids in some cases.
A provider inserts SPRINT’s thread-like wire through the skin near the nerve closest to the pain center and can be used for up to 60 days. The only item inside the body is the wire and an external stimulator is worn outside on a pad.
“At the end of treatment, it’s removed. And what we’ve found is it’s not only effective, there’s significant pain relief and improvement of the quality of life,” said SPR Therapeutics CEO Maria Bennett. “And for many, there’s a sustained effect after the device is removed.”
The FDA approved SPRINT for both acute and chronic pain. But at the moment, SPR is concentrating its efforts on fighting chronic pain, such as pain associated with knee replacement surgery, shoulder pain and neuropathic pain. Many patients will put off knee replacement for fear of post-surgical pain, and the likely need for prescription pain medication to find comfort during recovery, said Bennett.
SPRINT uses a neurostimulation platform, which has been used for decades for many different types of uses. Bennett said it’s one of the lowest invasive approaches to ease pain. However, it’s often leveraged on patches that focus on pain centers, but must go through the skin.
“It doesn’t get down to the root of the pain,” said Bennett.
Alternatively, there are neurostimulators, which are about the size of a stopwatch and surgically implanted. It delivers mild electrical signals in the epidural space near the spine by thin wires. While this is an incredibly effective method, it’s also expensive and invasive — making it a last resort treatment.
But SPRINT is designed to fill the gap in the middle of these treatment options, said Bennett.
“There are no tech options that are minimally invasive and with minimal cost to the provider or patient.”
“Opioids are filling that gap right now, so physicians and patients turn to opioids to deal with chronic pain. There isn’t another viable option,” she added. “We need something to map that pain. Opioids have served that purpose, but now we’re in crisis mode.”
SPR’s idea is to leverage less invasive tech, which providers can offer as an alternative to prescribing opioids. Bennett said there is no harm to patients to trying SPRINT to deal with pain, before opioids are considered. The most adverse event experienced by patients is skin irritation at the external stimulator placement.
The company launched its efforts at the beginning of this year, but Bennett said the hard part will be selling the device to hospitals, providers and pain centers. The device has been approved for use by several pain facilities, and the company is hoping to build from there.
Read the full story at Healthcare IT News.